Mathematics, 26.02.2020 00:15 josuemartinez1030
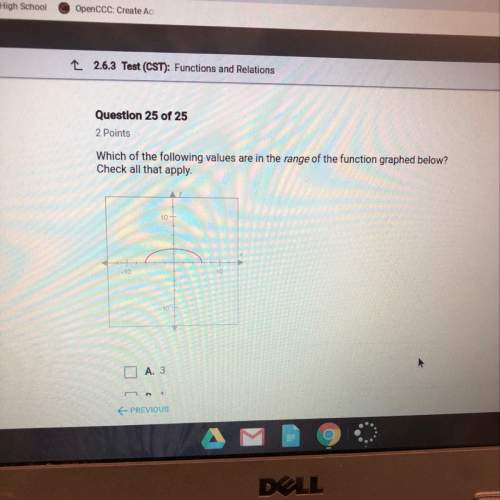
Calculate the specific heat of a metal (in calories/gram-degree C) from the following data. A container made of the metal has a mass of 3.77 kg and contains 18.1 kg of water. A 1.45 kg piece of the same metal, initially at a temperature of 164 degrees C, is placed in the water. The container and water initially have a temperature of 15 degrees C, and the final temperature of the entire system is 18 degrees C.

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 19:00
The focus of parabola is (-4, -5), and its directrix is y= -1. fill in the missing terms and signs in parabolas equation in standard form
Answers: 1

Mathematics, 21.06.2019 21:00
Yahir designs bracelets. he uses between 9 and 15 red and yellow beads in the ratio of 2 red bead to 5 yellow beads. drag the beads into the container to meet these requirements
Answers: 2

Mathematics, 21.06.2019 23:00
According to a study conducted in 2015, 18% of shoppers said that they prefer to buy generic instead of name-brand products. suppose that in a recent sample of 1500 shoppers, 315 stated that they prefer to buy generic instead of name-brand products. at a 5% significance level, can you conclude that the proportion of all shoppers who currently prefer to buy generic instead of name-brand products is higher than .18? use both the p-value and the critical-value approaches.
Answers: 1

Mathematics, 22.06.2019 00:00
At noon a tank contained 10cm water. after several hours it contained 7cm of water. what is the percent decrease of water in the tank?
Answers: 1
You know the right answer?
Calculate the specific heat of a metal (in calories/gram-degree C) from the following data. A contai...
Questions


Health, 11.05.2020 23:57







Mathematics, 11.05.2020 23:57


Computers and Technology, 11.05.2020 23:57


History, 11.05.2020 23:57

Mathematics, 11.05.2020 23:57



Mathematics, 11.05.2020 23:57

Chemistry, 11.05.2020 23:57


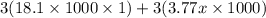 .
.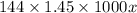 .
.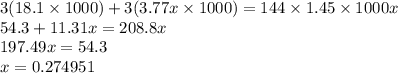 .
.