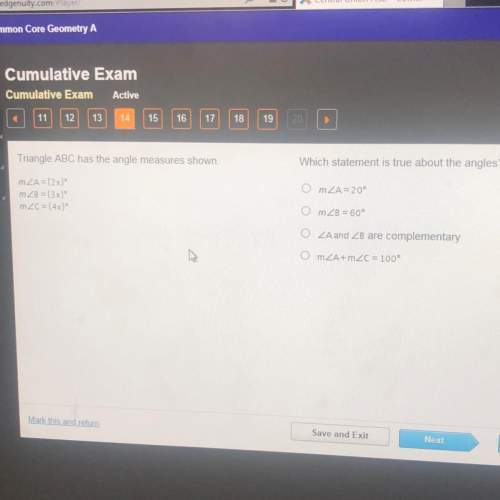
These diagrams show two atoms of fluorine and an atom of magnesium.
On the left, a purp...

Mathematics, 26.02.2020 23:15 aprilreneeclaroxob0c
These diagrams show two atoms of fluorine and an atom of magnesium.
On the left, a purple circle labeled F is shown twice, both times surrounded by 2 concentric circles. The inner circle has 2 small green spheres. The outer circle has 7 small green spheres. On the right, a purple circle labeled M g surrounded by 3 concentric circles. The inner circle has 2 small green spheres. The middle circle has 8 small green spheres. The outer circle has 1 small green sphere.
Which shows the correct steps in the formation of an ionic bond between these atoms?
A magnesium atom accepts six electrons from the fluorine atoms → Each fluorine atom donates three of the electrons → The magnesium atom becomes a -2 ion → Each fluorine atom becomes a +1 ion
A magnesium atom donates two electrons to the fluorine atoms → Each fluorine atom accepts one of the electrons → The magnesium atom becomes a -2 ion → Each fluorine atom becomes a +1 ion
A magnesium atom accepts two electrons from the fluorine atoms → Each fluorine atom donates one of the electrons → The magnesium atom becomes a -2 ion → Each fluorine atom becomes a +1 ion
A magnesium atom donates two electrons to the fluorine atoms → Each fluorine atom accepts one of the electrons → The magnesium atom becomes a +2 ion → Each fluorine atom becomes a -1 ion

Answers: 1
Another question on Mathematics


Mathematics, 21.06.2019 15:00
In a school 2/3 of the students study a language of those who study a language 2/5 study french
Answers: 2


Mathematics, 21.06.2019 21:30
Find the domain and range of the following function f(x) = 51x - 21+ 4
Answers: 2
You know the right answer?
Questions



Geography, 03.02.2021 21:10


Mathematics, 03.02.2021 21:10




Chemistry, 03.02.2021 21:10


Mathematics, 03.02.2021 21:10


Mathematics, 03.02.2021 21:10

Mathematics, 03.02.2021 21:10

Mathematics, 03.02.2021 21:10



Mathematics, 03.02.2021 21:10

Mathematics, 03.02.2021 21:10