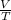Mathematics, 10.03.2020 19:13 andesmints5341
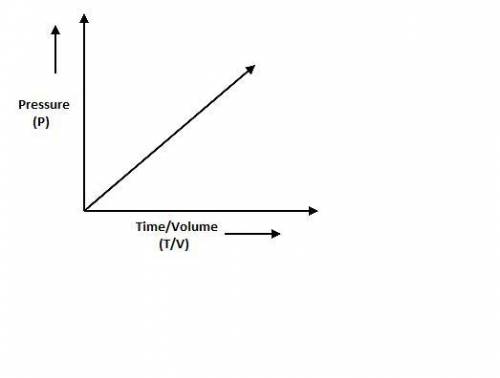
According to the Ideal Gas Law, PV = kT, where P is pressure, V is volume, T is temperature (in kelvins), and k is a constant of proportionality. A tank contains 2600 cubic inches of nitrogen at a pressure of 20 pounds per square inch and a temperature of 300 K.(a) Determine k. k = (b) Write P as a function of V and T and describe the level curves. P =Setting P = c, the level curves are of the formV =

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 16:00
The graph shows the function f(x) = (2.5)x was horizontally translated left by a value of h to get the function g(x) = (2.5)x–h.
Answers: 1

Mathematics, 21.06.2019 20:30
Kai walked one and two-thirds of a mile on saturday and four and one-sixth of a mile on sunday. how many miles did kai walk?
Answers: 1

Mathematics, 22.06.2019 01:00
Arrange the steps to solve this system of linear equations in the correct sequence. x + y = -2 2x – 3y = -9 tiles subtract 3x + 3y = -6 (obtained in step 1) from 2x – 3y = -9 (given) to solve for x. substitute the value of x in the first equation (x + y = -2) to get y = 1. the solution for the system of equations is (-3, 1). x = -15 the solution for the system of equations is (-15, 13). add 3x + 3y = -6 (obtained in step 1) to 2x – 3y = -9 (given), and solve for x. x = -3 substitute the value of x in the first equation (x + y = -2) to get y = 13. multiply the first equation by 3: 3(x + y) = 3(-2) 3x + 3y = -6.
Answers: 1

Mathematics, 22.06.2019 01:50
Felix wrote several equations and determined that only one of the equations has no solution. which of these equations has no solution?
Answers: 3
You know the right answer?
According to the Ideal Gas Law, PV = kT, where P is pressure, V is volume, T is temperature (in kelv...
Questions


Mathematics, 05.01.2021 01:00

Health, 05.01.2021 01:00


Mathematics, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00




History, 05.01.2021 01:00


Mathematics, 05.01.2021 01:00


Computers and Technology, 05.01.2021 01:00



Social Studies, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00

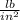
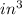
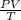
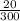 × 2600
× 2600