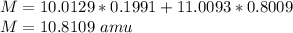Mathematics, 16.03.2020 23:47 oliviakate9230
Natural sample of boron consists of two isotopes. One has an exact mass of 10.0129 amu and its percent abundance is 19.91. The other isotope, of mass 11.0093 amu, has a percent abundance of 80.09. Calculate the average atomic mass.

Answers: 1
Another question on Mathematics


Mathematics, 21.06.2019 19:30
Which statements are true? check all that apply. the line x = 0 is perpendicular to the line y = –3. all lines that are parallel to the y-axis are vertical lines. all lines that are perpendicular to the x-axis have a slope of 0. the equation of the line parallel to the x-axis that passes through the point (2, –6) is x = 2. the equation of the line perpendicular to the y-axis that passes through the point (–5, 1) is y = 1.
Answers: 1

Mathematics, 21.06.2019 19:30
You have 17 cats that live in houses on your street, 24 dogs, 3 fish, and 4 hamsters. what percent of animals on your block are dogs?
Answers: 2

Mathematics, 21.06.2019 19:30
If you can solve all of these i will give ! - 4% of 190 - 4% of 162.5 - 4% of 140 - a 4% increase from 155.1 - a 4% increase from 159.8
Answers: 2
You know the right answer?
Natural sample of boron consists of two isotopes. One has an exact mass of 10.0129 amu and its perce...
Questions



History, 22.06.2019 12:30



Social Studies, 22.06.2019 12:30

History, 22.06.2019 12:30




Mathematics, 22.06.2019 12:30

Chemistry, 22.06.2019 12:30



Social Studies, 22.06.2019 12:30


History, 22.06.2019 12:30



Mathematics, 22.06.2019 12:30