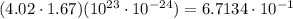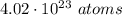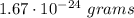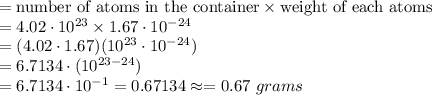Mathematics, 06.10.2019 07:01 ebt2367
Aclosed container has 4.02 ⋅ 1023 atoms of a gas. each atom of the gas weighs 1.67 ⋅ 10^−24 grams. which of the following shows and explains the approximate total mass, in grams, of all the atoms of the gas in the container?
6.71 grams, because (4.02 ⋅ 1.67) ⋅ (10^23 ⋅ 10^−24) = 6.7134
5.69 grams, because (4.02 + 1.67) ⋅ (10^23 ⋅ 10^−24) = 5.69
0.67 grams, because (4.02 ⋅ 1.67) ⋅ (10^23 ⋅ 10^−24) = 6.7134 ⋅ 10^−1
0.57 grams, because (4.02 + 1.67) ⋅ (10^23 ⋅ 10^−24) = 5.69 ⋅ 10^−1

Answers: 2
Another question on Mathematics


Mathematics, 21.06.2019 16:00
What kind of bond pays interest which is exempt from tax?
Answers: 1

Mathematics, 21.06.2019 20:50
What is the greatest number of parts of a circle that can be formed by cutting the circle with 7 straight cuts? (note: the parts do not have to be equal in size)
Answers: 3

You know the right answer?
Aclosed container has 4.02 ⋅ 1023 atoms of a gas. each atom of the gas weighs 1.67 ⋅ 10^−24 grams. w...
Questions



History, 23.11.2020 17:40

English, 23.11.2020 17:40

Mathematics, 23.11.2020 17:40



Mathematics, 23.11.2020 17:40

Mathematics, 23.11.2020 17:40


English, 23.11.2020 17:40

Mathematics, 23.11.2020 17:40

English, 23.11.2020 17:40

Law, 23.11.2020 17:40

Mathematics, 23.11.2020 17:40

English, 23.11.2020 17:40


Mathematics, 23.11.2020 17:40


Mathematics, 23.11.2020 17:40