NH3(g) + Cl2(g) -> NH4Cl(s)

Mathematics, 18.04.2020 01:43 amison64
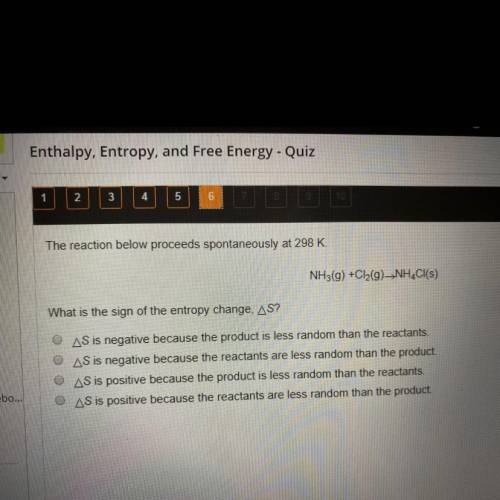
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
What is the sign of the entropy change, delta S?

Answers: 2
Another question on Mathematics

Mathematics, 21.06.2019 14:00
What is the slope of a line that is perpendicular to y = 3x + 1
Answers: 1

Mathematics, 21.06.2019 19:00
Marina baked cookies. she gave 3/4 of the cookies to the scout bake sale. she shared the remaining 6 cookies with her friends. how many cookies did marina bake?
Answers: 3

Mathematics, 21.06.2019 20:00
Put the equation in slope intercept form by solving for y
Answers: 2

Mathematics, 21.06.2019 20:30
Explain how you divide powers with like bases.discuss why the bases have to be the same.how are these rules similar to the rules for multiplying powers with like bases.
Answers: 1
You know the right answer?
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
NH3(g) + Cl2(g) -> NH4Cl(s)
Questions

Mathematics, 19.02.2020 03:52




Medicine, 19.02.2020 03:52

Health, 19.02.2020 03:52

English, 19.02.2020 03:53

Mathematics, 19.02.2020 03:53

Mathematics, 19.02.2020 03:53

History, 19.02.2020 03:53


Mathematics, 19.02.2020 03:53