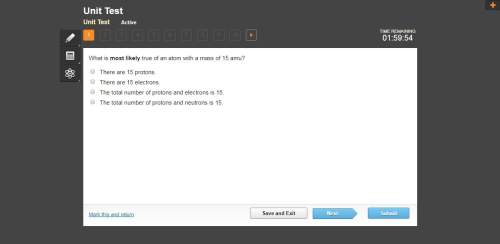
Mathematics, 05.05.2020 08:09 bossboybaker
Hund’s rule of maximum multiplicity specifies that . A. electrons will fill orbitals of lower energy first, pairing up only after each orbital of the same energy already has one electron B. electrons will fill orbitals of lower energy first, pairing up only after each s and p orbital has one electron C. electrons will fill all of the s orbitals before filling any p orbitals D. the pattern by which electrons occupy orbitals cannot be predicted

Answers: 1
Another question on Mathematics


Mathematics, 22.06.2019 00:50
Consider the enlargement of the pentagon. what is the value of x, rounded to the nearest tenth? 2.1 centimeters 3.3 centimeters 7.0 centimeters 15.0 centimeters
Answers: 3


Mathematics, 22.06.2019 03:00
An object is accelerating at a constant rate. its velocity in feet per second as a function of time in seconds can be modeled by the linear function v(t) = 2.5t. what does the dependent variable represent for this function? a) acceleration b) distance c) slope d) velocity
Answers: 3
You know the right answer?
Hund’s rule of maximum multiplicity specifies that . A. electrons will fill orbitals of lower energy...
Questions

Computers and Technology, 01.08.2019 08:00




History, 01.08.2019 08:00







Computers and Technology, 01.08.2019 08:00



Biology, 01.08.2019 08:00



Chemistry, 01.08.2019 08:00


Mathematics, 01.08.2019 08:00