The reaction below is at dynamic equilibrium.
N2(g) + 3H2(g) +
2NH3(g)
...

Mathematics, 07.05.2020 04:05 kaayla20
The reaction below is at dynamic equilibrium.
N2(g) + 3H2(g) +
2NH3(g)
Which statement is true for the equilibrium system?
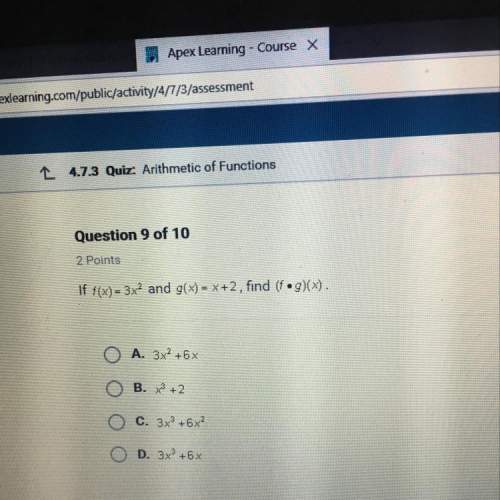
The concentration of NH, is greater than the concentration of N2.
The concentration of NH3 equals the concentration of N2.
The rate of the forward reaction equals the rate of the reverse reaction
The rate of the forward reaction is greater than the rate of the reverse reaction.

Answers: 2
Another question on Mathematics


Mathematics, 21.06.2019 17:00
One side of a rectangle is 7 feet shorter than seven times the other side. find the length of the shorter side if we also know that the perimeter of the rectangle is 306 feet.
Answers: 2

Mathematics, 21.06.2019 18:00
Clara schumann is buying bagels for her coworkers, she buys a dozen bagels priced at $5.49 a dozen. the bakery's cost for making the bagels is $2.25 per dozen. what is the markup rate based on selling price on a dozen bagels?
Answers: 1

Mathematics, 21.06.2019 18:10
Drag the tiles to the boxes to form correct pairs. not all tiles will be used. match each set of vertices with the type of quadrilateral they form.
Answers: 1
You know the right answer?
Questions

English, 23.06.2019 09:30

Chemistry, 23.06.2019 09:30

History, 23.06.2019 09:30

English, 23.06.2019 09:30

Computers and Technology, 23.06.2019 09:30

Biology, 23.06.2019 09:30



Mathematics, 23.06.2019 09:30


Mathematics, 23.06.2019 09:30



Mathematics, 23.06.2019 09:30

History, 23.06.2019 09:30





Advanced Placement (AP), 23.06.2019 09:30