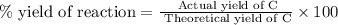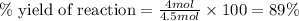Mathematics, 26.08.2019 10:50 purplefive85
Consider the following reaction: 2a + b -> 3c + d 3.0 mol a and 2.0 mol b react to form 4.0 mol c. what is the percent yield of this reaction?
a) 50%
b) 67%
c) 75%
d) 89%
e) 100%
what other formulas for % yield other than actual/theoretical yield?

Answers: 1
Another question on Mathematics


Mathematics, 21.06.2019 20:00
Aclothing store has the sign shown in the shop window. pani sees the sign and wants to buy 3 shirts and 2 pairs of jeans. the cost of each shirt before the discount is $12, and the cost of each pair of jeans is $19 before the discount. write and simplify an expression to find the amount pani pays if a $3 discount is applied to her total
Answers: 2

Mathematics, 21.06.2019 23:40
The frequency table shows the results of a survey asking people how many hours they spend online per week. on a piece of paper, draw a histogram to represent the data. then determine which answer choice matches the histogram you drew. in order here is the.. hours online: 0-3, 4-7, 8-11, 12-15, and 16-19. frequency: 5, 8, 10, 8, 7 answer for the question is in the picture! : )
Answers: 2

Mathematics, 22.06.2019 00:00
Given the diagram below, michael writes, "segment ac is congruent to segment ac." which of the following reasons allow him to write this statement?
Answers: 1
You know the right answer?
Consider the following reaction: 2a + b -> 3c + d 3.0 mol a and 2.0 mol b react to form 4.0 mol...
Questions





Mathematics, 27.01.2021 16:30






Mathematics, 27.01.2021 16:30




Physics, 27.01.2021 16:30



Mathematics, 27.01.2021 16:30

Social Studies, 27.01.2021 16:30

Chemistry, 27.01.2021 16:30

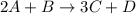
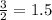 moles of B
moles of B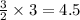 mole of C
mole of C