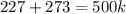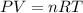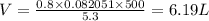Mathematics, 29.01.2020 07:58 bNicholson23
What is the volume of the container needed to store 0.8 moles of argon gas at 5.3 atm and 227°c? . (given: r = 0.08205 l · atm/mol · k). 2.81 liters. 4.39 liters. 6.19 liters. 9.67 liters

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 13:30
Lassify the function as linear or quadratic and identify the quadratic, linear, and constant terms. f(x) = (3x + 2)(−6x − 3) linear function; linear term: −21x; constant term: −6 linear function; linear term: −18x2; constant term: −6 quadratic function; quadratic term: 6x2; linear term: 24x; constant term: −6 quadratic function; quadratic term: −18x2; linear term: −21x; constant term: −6
Answers: 3

Mathematics, 21.06.2019 16:00
Apatient is to take 60 mg of an antibiotic on day 1, take 45mg on days 2 and 3, take 30mg on days 4 and 5 and take 15 mg on days 6 and 7. how many total mg will the patient take?
Answers: 1

Mathematics, 21.06.2019 19:30
What are the solutions to the following equation? |m| = 8.5 the value of m is equal to 8.5 and because each distance from zero is 8.5.
Answers: 3

Mathematics, 22.06.2019 01:30
Write 37/22 as a decimal rounded to the nearest hundredth.
Answers: 1
You know the right answer?
What is the volume of the container needed to store 0.8 moles of argon gas at 5.3 atm and 227°c? ....
Questions



Physics, 28.05.2021 18:30


History, 28.05.2021 18:30

Mathematics, 28.05.2021 18:30

Mathematics, 28.05.2021 18:30

Chemistry, 28.05.2021 18:30

History, 28.05.2021 18:30


Health, 28.05.2021 18:30




English, 28.05.2021 18:30


English, 28.05.2021 18:30


Chemistry, 28.05.2021 18:30