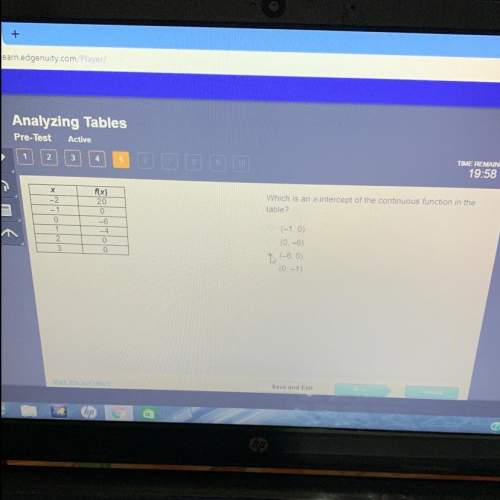
Mathematics, 04.07.2020 19:01 willia343
Methane, the chief component of natural gas, burns in oxygen according to the equation
CH4(g) + 2 O2(g) > CO2(g) + 2 H2O(g)
If 7.0 mol of CH4 react with an unlimited amount of oxygen, what amount (in moles) of H2O(g) will be produced?
please show your work!

Answers: 2
Another question on Mathematics

Mathematics, 21.06.2019 14:10
Determine whether the samples are independent or dependent. upper a data set includes the morning and evening temperature for the lasta data set includes the morning and evening temperature for the last 90 days.90 days.
Answers: 1

Mathematics, 21.06.2019 14:30
3. solve the given inequality and graph the solution on a number line.
Answers: 3

Mathematics, 21.06.2019 19:00
Twenty points. suppose that one doll house uses the 1 inch scale, meaning that 1 inch on the doll house corresponds to 1/2 foot for life-sized objects. if the doll house is 108 inches wide, what is the width of the full-sized house that it's modeled after? in inches and feet
Answers: 2

Mathematics, 21.06.2019 22:30
21 a stick 7 inches long is broken into two pieces, so that one piece is twice as long as the other one. how long are the two pieces?
Answers: 1
You know the right answer?
Methane, the chief component of natural gas, burns in oxygen according to the equation
CH4(g) + 2 O...
Questions

English, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31



Mathematics, 20.11.2019 20:31


Physics, 20.11.2019 20:31

Computers and Technology, 20.11.2019 20:31


History, 20.11.2019 20:31

Geography, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31

Health, 20.11.2019 20:31



Biology, 20.11.2019 20:31




Mathematics, 20.11.2019 20:31