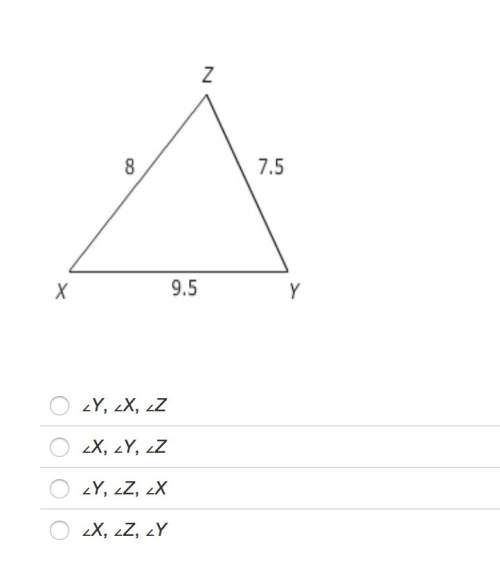
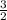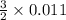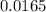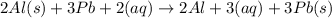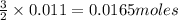Mathematics, 14.10.2019 11:00 carlo123
The equation for the reaction between al(s) and pb+2(aq) is:
2al(s) + 3pb+2(aq) 2al+2(aq) + 3pb(s)
therefore, for each 2 moles of al(s) that are used, how many moles of pb(s) are formed?
from the 0.011 mole of al(s) consumed, calculate the number of moles of pb(s) that should have been formed: mole(s)

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 19:00
Write a function for a rotation 90 degrees counter clockwise about the origin, point 0
Answers: 1

Mathematics, 21.06.2019 21:00
Estimate the area under the curve f(x) = 16 - x^2 from x = 0 to x = 3 by using three inscribed (under the curve) rectangles. answer to the nearest integer.
Answers: 1

Mathematics, 21.06.2019 22:00
If abcde is reflected over the x-axis and then translated 3 units left, what are the new coordinates d?
Answers: 3

Mathematics, 21.06.2019 22:30
How can constraints be used to model a real-world situation?
Answers: 1
You know the right answer?
The equation for the reaction between al(s) and pb+2(aq) is:
2al(s) + 3pb+2(aq) 2al+2(a...
2al(s) + 3pb+2(aq) 2al+2(a...
Questions


Mathematics, 31.08.2019 15:10

Geography, 31.08.2019 15:10

Mathematics, 31.08.2019 15:10

Arts, 31.08.2019 15:10


Chemistry, 31.08.2019 15:10



Mathematics, 31.08.2019 15:10

Biology, 31.08.2019 15:10


History, 31.08.2019 15:10


English, 31.08.2019 15:10



Mathematics, 31.08.2019 15:10