Mathematics, 16.10.2020 05:01 jose9794
[PLEASE ANSWER ASAP, ON TIME LIMIT!] The pH level of a substance can be determined by the formula pH = –log[H+], where [H+] is the hydrogen ion concentration in moles per liter. An alkaline substance has a pH level between 7 and 14. Which ion concentration corresponds to an alkaline substance? Answer choices in picture.
![[PLEASE ANSWER ASAP, ON TIME LIMIT!] The pH level of a substance can be determined by the formula p](/tpl/images/1832/4382/d471a.jpg)

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 19:50
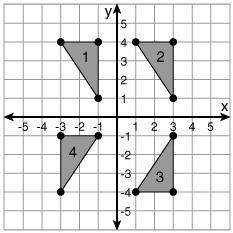
The graph shows the distance kerri drives on a trip. what is kerri's speed?
Answers: 3

Mathematics, 21.06.2019 23:30
Maren is buying carpet for her rectangular living room. the room is 4.8 yards wide and 5.2 yards long. how much carpet does she need to buy? enter your answer as a decimal in the box. yd2
Answers: 1

Mathematics, 22.06.2019 02:00
Arectangular yard has area 96 square feet. if the width of the yard is 4 feet less than the length
Answers: 1

Mathematics, 22.06.2019 02:20
Find the volume of the wedge cut from the first octant by the cylinder z=12-3y^2 and the plane x+y=2.
Answers: 1
You know the right answer?
[PLEASE ANSWER ASAP, ON TIME LIMIT!] The pH level of a substance can be determined by the formula pH...
Questions

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

English, 10.09.2020 22:01

Spanish, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Social Studies, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

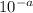
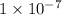 to
to 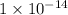 .
.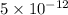 moles per liter lies in the given range.
moles per liter lies in the given range.