
Mathematics, 23.10.2020 21:30 lisaxo
I'm BEGGING u pls help...
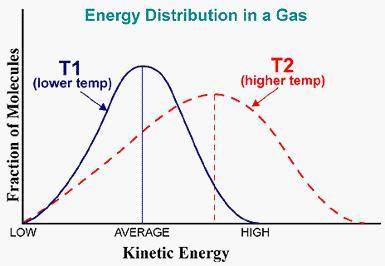
Apply the principles of Kinetic Molecular Theory to graphs of molecular motion This graph represents a population of molecules in a gas versus the distribution of the average velocity (speed) of its molecules in that population. Assume all molecules to be of the same mass. In reading the graph, it is important to note three things. One, is the most probable speed is at the peak of the curve. Secondly, the most probable speed increases as the temperature increases (so shift to the right), and the distribution broadens as it increases.
On the graph, indicate the average kinetic energy of the population at T1 and T2.
Which axis of the graph indicates the temperature of the sample?
Explain your On graph paper sketch a curve that represents the distribution of molecules at a temperature below the one shown. Label it as T3. Describe T1, and T3 in terms of their average kinetic energy. Be specific and detailed.

Answers: 2
Another question on Mathematics

Mathematics, 21.06.2019 13:30
Find the magnitude of the torque about p if an f = 80-lb force is applied as shown. (round your answer to the nearest whole number.) ft-lb
Answers: 1

Mathematics, 21.06.2019 14:30
Because of your favorite lemonade it is $3.84 for 3 gallons. write this as an unit rate
Answers: 2


Mathematics, 21.06.2019 23:20
Predict which statements are true about the intervals of the continuous function. check all that apply
Answers: 3
You know the right answer?
I'm BEGGING u pls help...
Apply the principles of Kinetic Molecular Theory to graphs of molecular m...
Questions

Geography, 08.07.2019 20:00

History, 08.07.2019 20:00

Biology, 08.07.2019 20:00


Mathematics, 08.07.2019 20:00

History, 08.07.2019 20:00




Mathematics, 08.07.2019 20:00

Mathematics, 08.07.2019 20:00



Spanish, 08.07.2019 20:00


History, 08.07.2019 20:00

History, 08.07.2019 20:00

History, 08.07.2019 20:00

Business, 08.07.2019 20:00



