
Mathematics, 02.11.2020 22:30 nsuleban5016
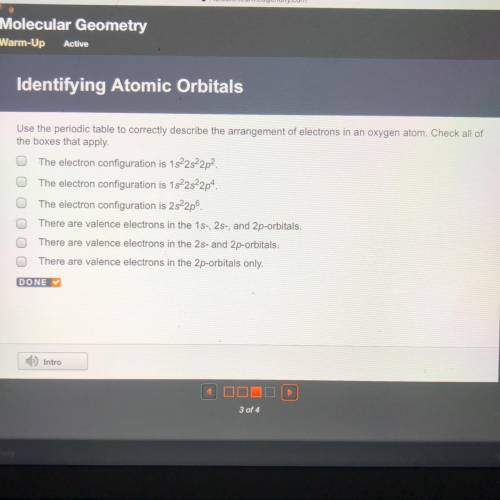
Use the periodic table to correctly describe the arrangement of electrons in an oxygen atom. Check all of
the boxes that apply.
The electron configuration is 1s22s22p2.
The electron configuration is 1s22s22p4.
The electron configuration is 2s22p6.
There are valence electrons in the 1s-, 2s-, and 2p-orbitals.
There are valence electrons in the 2s- and 2p-orbitals.
There are valence electrons in the 2p-orbitals only.

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 12:50
Amonument at a park is in the shape of a right square pyramid. a diagram of the pyramid and its net are shown. what is the surface area of the monument? enter the answer in the box. m²
Answers: 2

Mathematics, 21.06.2019 13:30
Apublic library wants to place 4 magazines and 9 books on each display shelf. the expressions 4s +9s represents the total number of items that will be displayed on s shelves. simplify this expression
Answers: 3

Mathematics, 21.06.2019 22:00
Identify the expression equivalent to 4(x + x + 7) − 2x + 8 − 4 by substituting x = 1 and x = 2.
Answers: 2

Mathematics, 21.06.2019 22:30
For the chance to be team captain, the numbers 1-30 are put in a hat and you get two chances to pick a number, without replacement. which formula correctly shows how to find the probability that you choose the number 1 and then 2?
Answers: 1
You know the right answer?
Use the periodic table to correctly describe the arrangement of electrons in an oxygen atom. Check a...
Questions

Mathematics, 24.02.2021 15:00

History, 24.02.2021 15:00




Health, 24.02.2021 15:00








Mathematics, 24.02.2021 15:00






Computers and Technology, 24.02.2021 15:00



