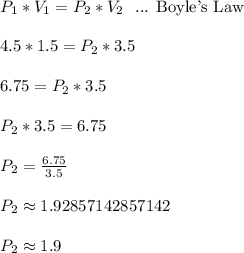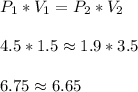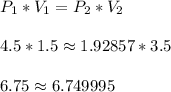Mathematics, 29.12.2020 20:30 ayoismeisalex
When a gas is kept at a constant temperature and pressure on it changes, its volume changes according to the following formula, known as Boyle’s law where P1 and V1 are the pressure (in atm) and the volume (in litres) at the beginning, and P2 and V2 are the pressure and the volume at the end. Find the final pressure P2 if V1 = 1.5 litres, P1 = 4.5 atm and V2 = 3.5 litres. Round to the nearest tenth of a atm.

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 17:30
The sales totals at macy's food store have increased exponentially over the months. which of these best shows the sales in the first three months?
Answers: 2

Mathematics, 21.06.2019 18:00
Express in the simplest form: (x^2+9x+14/x^2-49) / (3x+6/x^2+x-56)
Answers: 3

Mathematics, 21.06.2019 19:00
The diagonals of a quadrilaretral intersect at (-1,4). one of the sides of the quadrilateral is bounded by (2,7) and (-3,5) determine the coordinates of the other side in order for the quadrilaretral to be a square.
Answers: 1

You know the right answer?
When a gas is kept at a constant temperature and pressure on it changes, its volume changes accordin...
Questions


Mathematics, 04.05.2021 07:00


Chemistry, 04.05.2021 07:00

Biology, 04.05.2021 07:00

Mathematics, 04.05.2021 07:00





Mathematics, 04.05.2021 07:00

Physics, 04.05.2021 07:00

Mathematics, 04.05.2021 07:00

Advanced Placement (AP), 04.05.2021 07:00


Mathematics, 04.05.2021 07:00

English, 04.05.2021 07:00

Health, 04.05.2021 07:00


Mathematics, 04.05.2021 07:00