
Mathematics, 30.04.2021 19:50 Uhmjujiooo45701
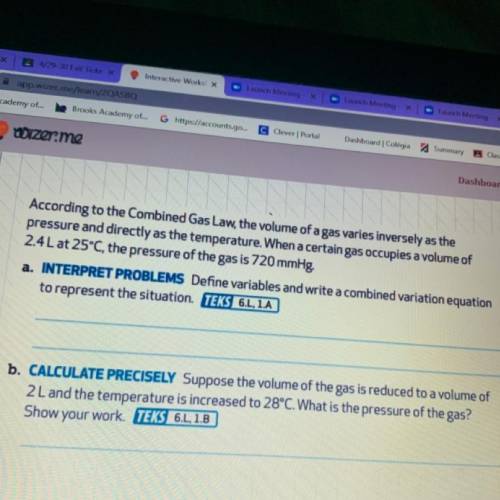
According to the Combined Gas Law, the volume of a gas varies inversely as the
pressure and directly as the temperature. When a certain gas occupies a volume of
2.4L at 25°C, the pressure of the gas is 720 mmHg?
a.
Define variables and write a combined variation equation
to represent the situation.
b. CALCULATE PRECISELY Suppose the volume of the gas is reduced to a volume of
2 L and the temperature is increased to 28°C. What is the pressure of the gas?
Show your work.

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 19:30
What is the effect on the graph of the function f(x)=x when f(x) is replaced with -f(x)+4
Answers: 1

Mathematics, 21.06.2019 20:30
Write an expression that represent sarah’s total pay last week.represent her hourly wage with w monday 5 tuesday 3 wednesday 0 noah hours wednesday 8 only with w wage did noah and sarah earn the same amount last week?
Answers: 3

Mathematics, 21.06.2019 22:00
5. (03.02)if g(x) = x2 + 3, find g(4). (2 points)1619811
Answers: 1

You know the right answer?
According to the Combined Gas Law, the volume of a gas varies inversely as the
pressure and direct...
Questions

Mathematics, 15.04.2021 17:40

Biology, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40



Mathematics, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40


History, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40

Mathematics, 15.04.2021 17:40


History, 15.04.2021 17:40


Mathematics, 15.04.2021 17:40




