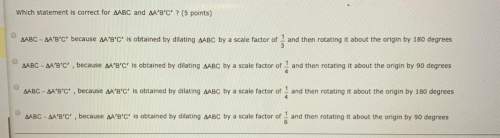
Mathematics, 13.09.2021 14:50 abolton04
A certain chemical reaction releases 301.kJ of heat energy per mole of reactant consumed. Suppose some moles of the reactant are put into a calorimeter (a device for measuring heat flow). It takes 2.81J of heat energy to raise the temperature of this calorimeter by 1°C. Now the reaction is run until all the reactant is gone, and the temperature of the calorimeter is found to rise by 10.4°C. How would you calculate the number of moles of reactant that were consumed?

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 20:00
Ialready asked this but i never got an answer. will give a high rating and perhaps brainliest. choose the linear inequality that describes the graph. the gray area represents the shaded region. y ≤ –4x – 2 y > –4x – 2 y ≥ –4x – 2 y < 4x – 2
Answers: 1


Mathematics, 21.06.2019 23:30
Which pair of numbers is relatively prime? a. 105 and 128 b. 130 and 273 c. 205 and 350 d. 273 and 333
Answers: 3

Mathematics, 22.06.2019 02:30
Below are two different functions, f(x) and g(x). what can be determined about their slopes? f(x)= −1x + 1 the function g(x) going through 0, 3 and 1, 1
Answers: 3
You know the right answer?
A certain chemical reaction releases 301.kJ of heat energy per mole of reactant consumed. Suppose so...
Questions

Geography, 25.09.2019 09:50

Health, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50


Social Studies, 25.09.2019 09:50




Spanish, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50

Social Studies, 25.09.2019 09:50


Mathematics, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50



Biology, 25.09.2019 09:50

English, 25.09.2019 09:50