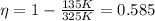Physics, 17.07.2019 09:30 alexkrol10
Aheat engine uses thermal energy that flows from a heat source with a temperature of 325 k to a cold sink, which has a temperature of 135 k. what is the efficiency of the heat engine? the answer should have three significant figures.

Answers: 2
Another question on Physics

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 11:40
Imagine that you have two balloons (or, better yet, actually inflate two balloons, if possible). create static electricity around one of the balloons by rubbing it against your hair or your sweater and then bring that balloon close to the other balloon, which has not been charged. try this with at least one other object—and for variety in the discussion, avoid using an object already described by your classmates. then, for your initial post to the discussion, answer the following questions: what happened with the two balloons?
Answers: 3

Physics, 22.06.2019 18:30
Suppose you plot the distance traveled by an object at various times and you discover that the graph is not a straight line. what does this indicate about the object's acceleration?
Answers: 3

Physics, 23.06.2019 00:30
3. which is not a primary color of light? blue red green yellow
Answers: 1
You know the right answer?
Aheat engine uses thermal energy that flows from a heat source with a temperature of 325 k to a cold...
Questions




Mathematics, 29.06.2019 15:40





Geography, 29.06.2019 15:40




Chemistry, 29.06.2019 15:40





Chemistry, 29.06.2019 15:40

Chemistry, 29.06.2019 15:40

Chemistry, 29.06.2019 15:40

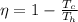
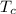 is the temperature of the cold sink
is the temperature of the cold sink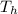 is the temperature of the heat source
is the temperature of the heat source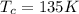 and
and 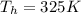 , so if we substitute these numbers into the equation, we find the efficiency of the engine:
, so if we substitute these numbers into the equation, we find the efficiency of the engine: