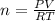Physics, 14.07.2019 16:30 keaton3410
According to avogadro's law, the volume of a gas is inversely related to the number of moles at constant temperature and pressure the volume of a gas is inversely related to the number of moles at standard temperature and pressure the volume of a gas depends only on the temperature and pressure the volume of a gas depends only on the number of moles in the sample the volume of a gas is directly related to the number of moles at constant temperature and pressure

Answers: 1
Another question on Physics

Physics, 21.06.2019 21:30
In what direction does the medium move relative to the direction of the wave? explain.
Answers: 3

Physics, 22.06.2019 02:30
Which type of bond is found between the atoms of a molecule? a. metallic b. delocalized electron c. covalent d. ionic
Answers: 1

Physics, 22.06.2019 04:10
Unpolarized light of intensity so passes through two sheets of polarizing material whose transmission axes make an angle of 60∘ with each other as shown in the figure. what is the intensity of the transmitted beam, st?
Answers: 3

Physics, 22.06.2019 07:30
Clothes dryer uses about 7 amps of current from a 240 volt line. how much power does it use?
Answers: 1
You know the right answer?
According to avogadro's law, the volume of a gas is inversely related to the number of moles at con...
Questions



Mathematics, 26.01.2022 06:30


Business, 26.01.2022 06:30


Physics, 26.01.2022 06:30

Mathematics, 26.01.2022 06:30




History, 26.01.2022 06:30



Social Studies, 26.01.2022 06:40



English, 26.01.2022 06:40