
Physics, 13.07.2019 13:00 cheyenne481
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water. when the jewelry is submerged in the graduated cylinder, the total volume increases to 61.2 ml. (a) determine the density of this piece of jewelry. (b) assuming that the jewelry is made from only one substance, what substance is it likely to be

Answers: 1
Another question on Physics

Physics, 22.06.2019 07:20
If the ama of the inclined plane below is 2, calculate the ima and efficiency. ima = efficiency =
Answers: 1

Physics, 22.06.2019 12:00
The sun’s mass is 2.0×10^ 30 kg, its radius is 7.0×10 5 km, and it has a rotational period of approximately 28 days. if the sun should collapse into a white dwarf of radius 3.5×10 3 km, what would its period be if no mass were ejected and a sphere of uniform density can model the sun both before and after?
Answers: 3

Physics, 22.06.2019 16:00
Two balls, each with a mass of 0.5 kg, collide on a pool table. is the law of conservation of momentum satisfied in the collision? explain why or why not
Answers: 1

Physics, 22.06.2019 19:10
3. a worker pushes a 30.5 kg polished stone along a polished table with a force on bonin a straight line for 5.20 s after starting from rest. there is no friction between thestone and the table. at the end of the table. the stone is hooked to a cable with a lengthof 62.0 cm to rotate it to a rough table to store until they can be picked up. the roughtable has a coefficient of static friction 0.702 with the polished stone. remember that 8= 9.80 m/s2a) what is the speed of the stone when it is hooked to the cable? b) what is the tension in the cable while the stone is rotating? (there is still nofriction)c) how much force is needed to make the stone begin sliding from rest on therough table? ius = 0.70262.0 cm30.5 kg20.5 kg6= 0
Answers: 2
You know the right answer?
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water...
Questions





English, 16.09.2019 20:00



Mathematics, 16.09.2019 20:00

History, 16.09.2019 20:00





Social Studies, 16.09.2019 20:00


Biology, 16.09.2019 20:00



English, 16.09.2019 20:00

Chemistry, 16.09.2019 20:00

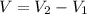
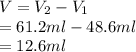
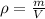
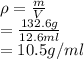
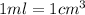 ,
,

