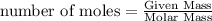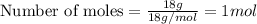Physics, 29.06.2019 22:00 jaidenkenna2001
Specific heat of water = 4.186 j g°c specific heat of ice = 2.00 j g°c molar heat of fusion = 6030 j mol molar heat of vaporization = 40790 j mol you take an ice cube (mass = 18g) from the freezer (t = -10°c) and place it on the table. later that day, you notice a puddle of water on the table that has reached ambient room temperature (20°c). how much heat must have been absorbed to make this happen?

Answers: 1
Another question on Physics

Physics, 21.06.2019 20:00
What are segments of dna that code for a particular trait. a combination of dna and proteins make up what
Answers: 1

Physics, 22.06.2019 01:30
An object of dimensions 50 cm x 40 cm x 0.20 cm has a mass 60g. find its density in g/cm3 and kg/ m3
Answers: 1

Physics, 22.06.2019 03:30
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change? work power gravitational energy chemical energy
Answers: 3

Physics, 22.06.2019 12:40
Question part points submissions used suppose that 2 j of work is needed to stretch a spring from its natural length of 26 cm to a length of 36 cm. (a) how much work is needed to stretch the spring from 28 cm to 32 cm? (round your answer to two decimal places.)
Answers: 2
You know the right answer?
Specific heat of water = 4.186 j g°c specific heat of ice = 2.00 j g°c molar heat of fusion = 6030...
Questions


Geography, 12.11.2019 21:31

Mathematics, 12.11.2019 21:31








Mathematics, 12.11.2019 21:31

Mathematics, 12.11.2019 21:31


Mathematics, 12.11.2019 21:31

Mathematics, 12.11.2019 21:31


Biology, 12.11.2019 21:31

History, 12.11.2019 21:31

History, 12.11.2019 21:31