
Physics, 29.06.2019 04:00 Christsflower601
What is the trend in sizes of the ions n3−, o 2−, f− and why does the trend exist? 1. n 3− > o 2− > f −; the effective nuclear charge is smallest at n3− and the largest at f −. 2. n 3− > o 2− > f −; for an isoelelectronic series, the ionic size decreases as the p + e− ratio decreases. 3. impossible to tell, since ionic radii for different elements cannot be compared; ions can only be compared to the neutral atom. 4. f − > o 2− > n 3−; in an isoelelectronic se-?

Answers: 1
Another question on Physics

Physics, 22.06.2019 17:40
You throw a baseball directly upward at time =0 at an initial speed of 14.9 m/s. what is the maximum height the ball reaches above where it leaves your hand? ignore air resistance and take =9.80 m/s2.
Answers: 1

Physics, 22.06.2019 21:00
How is the acceleration of an object in uniform circular motion constant? a - in magnitude only b - in direction only c - in both magnitude and direction d - in magnitude, direction & orientation
Answers: 3

Physics, 22.06.2019 21:30
Contrast the force of gravity between these pairs of objects: 1-kg mass and a 2-kg mass that are 1 m apart; a 1-kg mass and a 2-kg mass that are 2 m apart; and two 2=kg masses that are 1 m apart. i dont understand.
Answers: 3

Physics, 22.06.2019 22:00
It takes a lot of energy to get the temperature of water to increase and eventually boil because water has a high heat.
Answers: 1
You know the right answer?
What is the trend in sizes of the ions n3−, o 2−, f− and why does the trend exist? 1. n 3− > o...
Questions

History, 08.12.2020 06:50

Computers and Technology, 08.12.2020 06:50

Chemistry, 08.12.2020 06:50

SAT, 08.12.2020 06:50


Biology, 08.12.2020 06:50


Mathematics, 08.12.2020 06:50

Biology, 08.12.2020 06:50

History, 08.12.2020 06:50




Physics, 08.12.2020 06:50

Mathematics, 08.12.2020 06:50


Mathematics, 08.12.2020 06:50

History, 08.12.2020 06:50


Biology, 08.12.2020 06:50

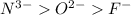 ; the effective nuclear charge is smallest at
; the effective nuclear charge is smallest at 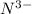 and the largest at
and the largest at 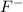
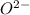 : Atomic number or number of electrons or number of protons in Oxygen is 8. As
: Atomic number or number of electrons or number of protons in Oxygen is 8. As 

