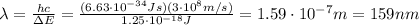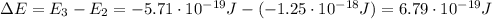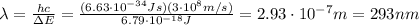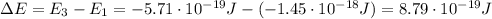Physics, 20.09.2019 10:30 JuanTorres7
Be sure to answer all parts. consider the following energy levels of a hypothetical atom: e4 −2.01 × 10−19 j e3 −5.71 × 10−19 j e2 −1.25 × 10−18 j e1 −1.45 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? × 10 m (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? × 10 j (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process. × 10 m

Answers: 1
Another question on Physics

Physics, 22.06.2019 12:30
When a vertical beam of light passes through a transparent medium, the rate at which its intensity i decreases is proportional to i(t), where t represents the thickness of the medium (in feet). in clear seawater, the intensity 3 feet below the surface is 25% of the initial intensity i0 of the incident beam. what is the intensity of the beam "10" feet below the surface? (give your answer in terms of i0. round any constants or coefficients to five decimal places.)
Answers: 2

Physics, 22.06.2019 22:00
Ne way in which elements differ from each other is the structure of the electron cloud in each element's atoms. in an electron cloud, an electron that is farther away from the nucleus has
Answers: 1

Physics, 22.06.2019 22:50
What is an electric motor? explain its operation. 2-3 sentence
Answers: 1

You know the right answer?
Be sure to answer all parts. consider the following energy levels of a hypothetical atom: e4 −2.01...
Questions


English, 30.03.2021 21:20

Social Studies, 30.03.2021 21:20

Mathematics, 30.03.2021 21:20


English, 30.03.2021 21:20



Biology, 30.03.2021 21:20


History, 30.03.2021 21:20







History, 30.03.2021 21:30


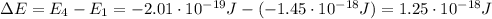
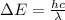
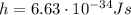 is the Planck constant
is the Planck constant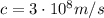 is the speed of light
is the speed of light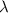 is the wavelength of the photon
is the wavelength of the photon