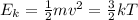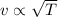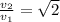Physics, 04.02.2020 14:54 smiley29162
Which of the following statements is not a correct assumption of the classicalmodel of an ideal gas? a. the molecules are in random motion. b. the volume of the molecules is negligible compared with the volume occupied bythe gas. c. the molecules obey newton's laws of motion. d. the collisions between molecules are inelastic. e. the only appreciable forces on the molecules are those that occur duringcollisions. a sample of an ideal gas is in a tank of constant volume. the sample absorbsheat energy so that its temperature changes from 300 k to 600 k. if v1 is theaverage speed of the gas molecules before the absorption of heat and v2 is theiraverage speed after the absorption of heat, what is the ratio v2/ v1? a. 1/2 b. 1 c. 2 d. 2 e. 4

Answers: 3
Another question on Physics


Physics, 22.06.2019 11:00
The star betelgeuse is about 600 light-years away. if it explodes tonight, a. we'll know because debris from the explosion will rain down on us from space.b. we'll know it immediately because it will be brighter than the full moon in the sky.c. we won't know about it until 600 years from now.d. none of the above.
Answers: 3

Physics, 22.06.2019 11:00
What is the rate of 12 liters of water moving through a water hose in 4.0 minutes?
Answers: 1

Physics, 22.06.2019 17:00
The force it would take to accelerate an 900-kg car at the rate of 6m/s2
Answers: 1
You know the right answer?
Which of the following statements is not a correct assumption of the classicalmodel of an ideal gas?...
Questions

Mathematics, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57

Biology, 05.05.2020 04:57

Social Studies, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57


History, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57







Mathematics, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57


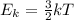
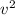 :
: