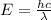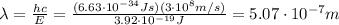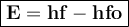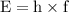Physics, 26.10.2019 16:43 acavalieri72
Image that the radiation emitted by the nitrogen at a frequency of 8.88×1014 hz is absorbed by an electron in a molecule of methyl salicylate. as a result, the electron in the wintergreen oil molecule jumps to an excited state. before returning to its ground state, the electron drops to an intermediate energy level, releasing two-thirds of the energy previously absorbed and emitting a photon. what is the wavelength of the photon emitted by the wintergreen oil molecule?

Answers: 1
Another question on Physics

Physics, 21.06.2019 18:20
Find the vertex(use the completing the square method), findthe x- and y-intercepts, and sketch the graph offunction. y = 2x2 + 4x - 1
Answers: 3

Physics, 22.06.2019 04:20
Astone is thrown into a pond. what happens to the amplitude of the resulting waves as they get farther from the point where the stone hit the water? explain.
Answers: 3

Physics, 22.06.2019 05:30
What do you think car designers do if the damage caused by a crash test is too severe?
Answers: 1

Physics, 22.06.2019 08:00
What is a carrier wave and how does it affect what you hear on the radio
Answers: 1
You know the right answer?
Image that the radiation emitted by the nitrogen at a frequency of 8.88×1014 hz is absorbed by an el...
Questions




Mathematics, 15.01.2021 23:30

Arts, 15.01.2021 23:30


English, 15.01.2021 23:30


Physics, 15.01.2021 23:30

History, 15.01.2021 23:30

Mathematics, 15.01.2021 23:30

Mathematics, 15.01.2021 23:30


Mathematics, 15.01.2021 23:30


Biology, 15.01.2021 23:30


Social Studies, 15.01.2021 23:30

Mathematics, 15.01.2021 23:30

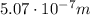 (507 nm)
(507 nm)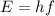
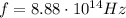 is the frequency of the photon
is the frequency of the photon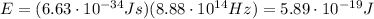
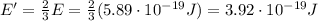
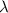 is
is