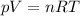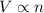Physics, 27.09.2019 12:00 Ryantimes2
Which of the following laws concerning gases is misstated? a. in a mixture of gases, the total pressure of the gases equals the sum of the individual pressures of each gas. b. at fixed temperature and pressure, the volume of a gas is inversely proportional to the number of moles of that gas. c. at fixed pressure, the volume of a gas is directly proportional to its temperature in kelvin. d. at fixed temperature, the volume of a gas is inversely proportional to its pressure. e. the average translational kinetic energy of the molecules of a gas is directly proportional to the temperature in kelvin.

Answers: 1
Another question on Physics

Physics, 22.06.2019 10:30
You are given two vectors a⃗ =−3.00ι^ 5.00j^ and b⃗ =5.00ι^ 2.00j^. let the counterclockwise angles be positive.
Answers: 3

Physics, 22.06.2019 19:30
Acamcorder has a power rating of 20 watts. if the output voltage from its battery is 9 volts, what current does it use?
Answers: 2


Physics, 23.06.2019 08:00
Stephanie lives in california, and her cousin thomas lives in france. which coordinate system would be most useful if the two wanted to observe the same meteor shower and discuss their observations over online chat as they're making them? a. horizontal b. equatorial c. azimuth-altitude d. celestial or azimuth-altitude
Answers: 1
You know the right answer?
Which of the following laws concerning gases is misstated? a. in a mixture of gases, the total pres...
Questions

World Languages, 03.11.2020 17:50



Mathematics, 03.11.2020 17:50


Engineering, 03.11.2020 17:50





Chemistry, 03.11.2020 17:50


History, 03.11.2020 17:50




Mathematics, 03.11.2020 17:50


Chemistry, 03.11.2020 17:50