
Physics, 29.01.2020 20:53 lanipooh01
The kinetic molecular theory of gases learning goal to understand some aspects of molecular motion in the gas phase the kinetic molecular theory of gases explains how gas molecules behave in terms of motion, speed, and energy one important aspect of this theory deals with the relationship between temperature and the average speed of the gas molecules. increasing the temperature of a gas sample increases the average kinetic energy of the molecules. the kinetic energy of a molecule determines its speed it is important to realize that not all molecules in a sample will have the same kinetic energy, which is why we refer to the average kinetic energy and the average speed. the speed of a particle with average kinetic energy is called the root mean square (rms) speed, vrms the rms speed may be expressed by the following equation: vrms=√3rt/mwhere r is the ideal gas constant, t is the absolute temperature, and m is the molar mass of the substance in kilograms per mole the constant motion of gas molecules causes diffusion and effusion. diffusion is the gradual mixing of two substances resulting from the movement of their particles. effusion is the gradual escape of gas molecules through microscopic holes in their container. part a which of the following state ments are true? check all that apply the average kinetic energy of gas molecules increases with increasing temperature there are gas molecules that move faster than the average the temperature of a gas sample is independent of the average kinetic energy the average speed of gas molecules decreases with decreasing temperature all the gas molecules in a sample cannot have the same kinetic energy

Answers: 3
Another question on Physics

Physics, 22.06.2019 07:30
Gas cloud 1 is likely to form a star. gas cloud 2 is not. based on this information, match the given conditions with each cloud
Answers: 2

Physics, 22.06.2019 12:30
Consider a system with two masses that are moving away from each other. why will the kinetic energy differ if the frame of reference is a stationary observer or one of the masses?
Answers: 1

Physics, 22.06.2019 13:20
This energy transformation diagram represents the energy of a skateboarder moving along a half-pipe. as she skates toward the top of the half-pipe, her original kinetic energy is converted to potential energy and friction. how much of the energy is potential?
Answers: 3

Physics, 22.06.2019 16:00
While the change in blank will remain the same during a collision, the force needed to bring an object to a stop can be blank if the time if collision is blank
Answers: 1
You know the right answer?
The kinetic molecular theory of gases learning goal to understand some aspects of molecular motion i...
Questions


Mathematics, 05.11.2020 09:40




Mathematics, 05.11.2020 09:40



English, 05.11.2020 09:40

Mathematics, 05.11.2020 09:40




Computers and Technology, 05.11.2020 09:40



Mathematics, 05.11.2020 09:40



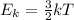 (1)
(1)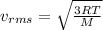 (2)
(2)

