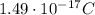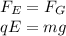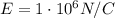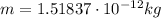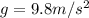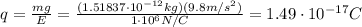Physics, 26.01.2020 23:31 moneybabyy38
In 1909 robert millikan was the first to find the charge of an electron in his now-famous oil drop experiment. in the experiment tiny oil drops are sprayed into a uniform electric field between a horizontal pair of oppositely charged plates. the drops are observed with a magnifying eyepiece, and the electric field is adjusted so that the upward force q e on some negatively charged oil drops is just sufficient to balance the downward force m g of gravity. millikan accurately measured the charges on many oil drops and found the values to be whole-number multiples of 1.6 × 10−19 c — the charge of the electron. for this he won the nobel prize. if a drop of mass 1.51837 × 10−12 kg remains stationary in an electric field of 1 × 106 n/c, what is the charge on this drop? the acceleration due to gravity is 9.8 m/s 2 . answer in units of c.

Answers: 2
Another question on Physics

Physics, 21.06.2019 20:00
What happens to atoms and chemical bonds during a reaction?
Answers: 1

Physics, 22.06.2019 01:00
Velocity is a description of both speed and direction, therefore it - a vector - a force arrow - the same as acceleration - a magnitude
Answers: 1

Physics, 22.06.2019 05:40
An ideal polarizer with its transmission axis rotated 30 degrees to the vertical is placed in a beam of unpolarized light of intensity 10w/m^2. after passing through the polarizer, what is the intensity of the beam? a. 8.7 w/m^2 b. 7.5 w/m^2 c. 5.0 w/m^2 d. 10 w/m^2 e. 2.5 w/m^2
Answers: 1

Physics, 22.06.2019 07:30
Examine the nuclear reacti why is this classified as a nuclear reaction rather than a chemical reaction? it is not balanced. a new compound is formed. a change has occurred in a nucleus. a new element has been formed.
Answers: 2
You know the right answer?
In 1909 robert millikan was the first to find the charge of an electron in his now-famous oil drop e...
Questions

History, 01.06.2021 17:20



Physics, 01.06.2021 17:20

Mathematics, 01.06.2021 17:20



Mathematics, 01.06.2021 17:20



Social Studies, 01.06.2021 17:20


Mathematics, 01.06.2021 17:20

Business, 01.06.2021 17:20

Mathematics, 01.06.2021 17:20

Mathematics, 01.06.2021 17:20



Physics, 01.06.2021 17:20