
Physics, 03.07.2019 22:10 Carrchris021
The temperature of a pot of water is 90.0 °c and the mass of the water is 112 g. a block with a mass of 21.0 g and a temperature of 20.0°c is dropped into the water. the final temperature of the water and the block is 88.3 °c. the specific heat of water is exactly 1 cal/g.°c. how much heat was lost by the water? o 1.90 x10^2 cal o 35.7 cal o 7650 cal o 1.70 cal how much heat was gained by the block? o 1430 cal o 190 x10^2 cal o 35.7 cal o 68.3 cal what is the specific heat of the block? o 0.133 cal/g.°c o 5.33 cal/g.°c o273000 cal/g.°c o0.0249 cal/g.°c

Answers: 2
Another question on Physics



Physics, 22.06.2019 16:30
Which magnetic property best describes a magnet’s ability to act at a distance? magnets are dipolar. magnets attract only certain objects. magnets have magnetic fields. magnets can transfer their properties to certain materials.
Answers: 1

Physics, 23.06.2019 01:30
In general what determines whether atoms will form chemical bonds?
Answers: 2
You know the right answer?
The temperature of a pot of water is 90.0 °c and the mass of the water is 112 g. a block with a mass...
Questions


History, 29.12.2019 23:31

Computers and Technology, 29.12.2019 23:31

Biology, 29.12.2019 23:31


English, 29.12.2019 23:31


Mathematics, 29.12.2019 23:31

History, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31

History, 29.12.2019 23:31







 = mass of water = 112 g
= mass of water = 112 g  = specific heat of water = 1 cal/(g °C)
= specific heat of water = 1 cal/(g °C) = initial temperature of water = 90.0 °C
= initial temperature of water = 90.0 °C = final temperature of water = 88.3 °C
= final temperature of water = 88.3 °C = Heat lost by water
= Heat lost by water 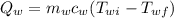
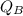 = Heat gained by the block
= Heat gained by the block  = mass of block = 21.0 g
= mass of block = 21.0 g  = specific heat of block
= specific heat of block  = initial temperature of block = 20.0 °C
= initial temperature of block = 20.0 °C = final temperature of block = 88.3 °C
= final temperature of block = 88.3 °C

