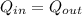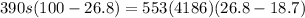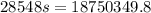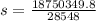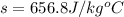Physics, 03.07.2019 22:30 codyshs160
An unknown metal alloy with a mass of 390 g is taken from boiling water and dropped into an insulated cup that contains 553 g of water at an initial temperature of 18.7°c. the final temperature of the system is 26.8°c. what is the metal's specific heat capacity?

Answers: 3
Another question on Physics

Physics, 22.06.2019 06:30
Which features on mars point to the possibility of liquid water on the planet? impact craters with sharp rims volcanic cones with craters gullies and stream-like channels mountain ranges with faults
Answers: 1

Physics, 22.06.2019 13:50
9.98 kg of r-134a at 300 kpa fills a rigid container whose volume is 14 l. determine the temperature and total enthalpy in the container. the container is now heated until the pressure is 600 kpa. determine the temperature and total enthalpy when the heating is completed. use data from the steam tables.
Answers: 1

Physics, 22.06.2019 15:00
Consider a uniformly charged ring in the xy plane, centered at the origin. the ring has radius a and positive charge qdistributed evenly along its circumference. a)what is the direction of the electric field at any point on the z axis? . b)what is the magnitude of the electric field along the positive z axis? use k in your answer, where k=14πϵ0. d)the ball will oscillate along the z axis between z=d and z=−d in simple harmonic motion. what will be the angular frequency ω of these oscillations? use the approximation d≪a to simplify your calculation; that is, assume that d2+a2≈a2. express your answer in terms of given charges, dimensions, and constants
Answers: 2

Physics, 22.06.2019 15:30
The voltage applied across a given parallel-plate capacitor is doubled. how is the energy stored in the capacitor affected?
Answers: 2
You know the right answer?
An unknown metal alloy with a mass of 390 g is taken from boiling water and dropped into an insulate...
Questions



Mathematics, 28.11.2019 23:31

History, 28.11.2019 23:31

History, 28.11.2019 23:31




Biology, 28.11.2019 23:31



History, 28.11.2019 23:31

Mathematics, 28.11.2019 23:31


Biology, 28.11.2019 23:31

Mathematics, 28.11.2019 23:31



Biology, 28.11.2019 23:31