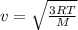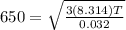Answers: 2
Another question on Physics

Physics, 21.06.2019 14:10
Asteady current of 1 ais flowing through a conducter calculate the number of electrons that flows through conductor in 1second
Answers: 1

Physics, 21.06.2019 20:00
When using two-wire cable to feed a 240-v appliance that does not require a neutral wire, you should a. mark both ends of the white wire green. b. use the cable as is. c. use only the black wire. d. mark both ends and any exposed area of the white wire black.
Answers: 1

Physics, 22.06.2019 02:30
Mass (kg) force (n) 5 25 10 50 15 75 20 100 a student was trying to find the relationship between mass and force. he placed four different masses on a table and pulled them using a spring scale. the table shows the different masses used in the experiment and the force required to pull each mass. the student concluded that more force was required to pull heavier objects. what comment would you make regarding his conclusion? a) no clear relation can be observed between mass and force from the data. b) there is a direct proportion between the mass and force listed in the table. c) gravity should have been taken into account while performing the experiment. d) there is an inverse proportion between the mass and force listed in the table.
Answers: 2

Physics, 22.06.2019 10:30
An insulated 40 ft^3 rigid tank contains air at 50 psia and 120°f. a valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 25 psia. the air temperature during this process is kept constant by an electric resistance heater placed in the tank. determine the electrical work done during this process.
Answers: 2
You know the right answer?
The rms speed of an oxygen molecule (o2) in a container of oxygen gas is 650 m/s. what is the temper...
Questions


History, 22.08.2019 19:00

Mathematics, 22.08.2019 19:00

Mathematics, 22.08.2019 19:00

Chemistry, 22.08.2019 19:00


English, 22.08.2019 19:00



Physics, 22.08.2019 19:00

Geography, 22.08.2019 19:00



Geography, 22.08.2019 19:00

English, 22.08.2019 19:00


Physics, 22.08.2019 19:00

Social Studies, 22.08.2019 19:00

Health, 22.08.2019 19:00