
Physics, 03.07.2019 23:30 itaheart101
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorimeter at a temperature of 22°c. determine the final temperature of the system consisting of the ice, water, and calorimeter. remember that the ice must first warm to 0°c, melt, and then continue warming as water. the specific heat of ice is 0.500 cal/g·°c = 2090 j/kg°c.

Answers: 1
Another question on Physics

Physics, 22.06.2019 00:30
Order the sequence of ideas that lead to marie curies discovery of radioactive elements number the events in chronological order starting with the oldest
Answers: 2

Physics, 22.06.2019 15:20
What component of earth’s atmosphere exists entirely as a result of photosynthesis?
Answers: 2


Physics, 22.06.2019 16:00
Two balls, each with a mass of 0.5 kg, collide on a pool table. is the law of conservation of momentum satisfied in the collision? explain why or why not
Answers: 1
You know the right answer?
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorime...
Questions

Mathematics, 12.02.2020 08:53

Mathematics, 12.02.2020 08:54

Mathematics, 12.02.2020 08:55


Mathematics, 12.02.2020 08:55

Computers and Technology, 12.02.2020 08:55


Mathematics, 12.02.2020 08:59


Mathematics, 12.02.2020 09:00







Mathematics, 12.02.2020 09:13

Biology, 12.02.2020 09:13

Mathematics, 12.02.2020 09:13

Social Studies, 12.02.2020 09:13

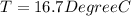
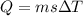
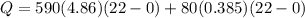
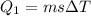
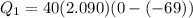
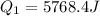
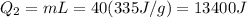
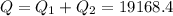
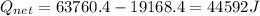
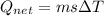
![44592 = (590 + 40)(4.186)(T - 0) + 80(0.385)(T - 0)[tex]T = 16.7 Degree C](/tpl/images/0048/1109/c6b32.png)


