
Physics, 05.07.2019 03:20 0Brittany0
Suppose 8.50 ✕ 10^5 j of energy are transferred to 1.63 kg of ice at 0°c. the latent heat of fusion and specific heat of water are lf = 3.33 ✕ 105 j/kg and c = 4186 j (kg · °c) . hint (a) calculate the energy (in j) required to melt all the ice into liquid water. (enter your answer to at least three significant figures.) j (b) how much energy (in j) remains to raise the temperature of the liquid water? (enter your answer to at least three significant figures.) j (c) determine the final temperature of the liquid water in celsius. °c

Answers: 3
Another question on Physics

Physics, 22.06.2019 04:00
Assume similar data for the motion of the blood in your aorta. estimate how many beats of the heart it will take the blood to get from your heart to your brain. (assume that the distance from your heart to your brain is 30 cm)
Answers: 2

Physics, 22.06.2019 15:20
Your science teacher brings in a speaker to talk to your class about climate change. during the session, students ask a few questions. which questions are related to the current evidence on climate change
Answers: 3

Physics, 22.06.2019 17:30
Ethanol has a heat of vaporization of 38.56 kj/mol and a normal boiling point of 78.4 c. what is the vapor pressure of ethanol at 14 c?
Answers: 3

Physics, 22.06.2019 18:00
Astudent pushes a 60-n block across the floor for a distance of 10 m. how much work was done to move the block
Answers: 1
You know the right answer?
Suppose 8.50 ✕ 10^5 j of energy are transferred to 1.63 kg of ice at 0°c. the latent heat of fusion...
Questions

Mathematics, 31.03.2021 20:00



Mathematics, 31.03.2021 20:00

Biology, 31.03.2021 20:00


Physics, 31.03.2021 20:00

Mathematics, 31.03.2021 20:00



Biology, 31.03.2021 20:00




Law, 31.03.2021 20:00



Mathematics, 31.03.2021 20:00

Mathematics, 31.03.2021 20:00

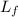 = Latent heat of fusion of ice to water = 3.33 x 10⁵ J/kg
= Latent heat of fusion of ice to water = 3.33 x 10⁵ J/kg 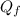 = Energy required to melt the ice
= Energy required to melt the ice 

