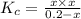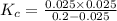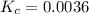Physics, 18.07.2019 21:20 einstein101
If 0.40 moles of pcl5 is heated in a 10.0 l container, equilibrium is established in which 0.25 moles of cl2 are present. the reaction is: pcl5(g)\longleftrightarrow ⟺ pcl3(g) + cl2(g) what is the value of the equilibrium constant?

Answers: 1
Another question on Physics

Physics, 22.06.2019 05:00
Aperson standing in a canoe exerts a force of 700 n to throw an anchor over the side. find the acceleration of the canoe if the total mass of the canoe and the person is 100 kg?
Answers: 1

Physics, 22.06.2019 09:30
Asap i'm in class rn a 1,000-kg car is traveling 20 m/s on a flat stretch of road. it gets to a hill and coasts uphill until it stops. how high up the hill does the car travel? givens: equation: 1/2mv2initial=mghfinal solve for h. plug & chug, label.
Answers: 2

Physics, 22.06.2019 15:00
Lightning is an example of what phenomenon? a release of a large amount of energy an absorption of a large amount of energy a natural electric circuit a natural electric current
Answers: 1

Physics, 22.06.2019 16:30
Acoil suspended freely, points in some direction when no current is passed through it . can you tell what will happen when a current is passed though it?
Answers: 3
You know the right answer?
If 0.40 moles of pcl5 is heated in a 10.0 l container, equilibrium is established in which 0.25 mole...
Questions

Mathematics, 27.07.2019 11:30

Social Studies, 27.07.2019 11:30




Mathematics, 27.07.2019 11:30


History, 27.07.2019 11:30


Mathematics, 27.07.2019 11:30


Biology, 27.07.2019 11:30



Social Studies, 27.07.2019 11:30

Biology, 27.07.2019 11:30

Business, 27.07.2019 11:30



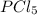 = 2 mole
= 2 mole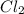 at equilibrium= 0.25 mole
at equilibrium= 0.25 mole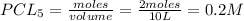
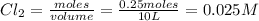
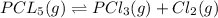
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/0105/4048/ffe89.png)