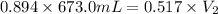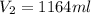Physics, 19.07.2019 01:10 conyabrew82
Agas occupying a volume of 673.0 ml at a pressure of 0.894 atm is allowed to expand at constant temperature until its pressure reaches 0.517 atm. what is its final volume?

Answers: 3
Another question on Physics

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 04:30
Ameter stick is pivoted at the 0.50-m line. a 3.0-kg object is hung from the 0.15-m line. where should a 5.0-kg object be hung to achieve equilibrium (the meter stick oriented horizontal and motionless)?
Answers: 1

Physics, 22.06.2019 05:30
Which of the choices below is one of the primary gases found in the atmosphere? a. helium b. carbon dioxide c. nitrogen d. argon
Answers: 2

Physics, 22.06.2019 08:00
What is the average speed of a car that travels 40mph for 1 hour and 60 mph in another hour will mark brainliest
Answers: 1
You know the right answer?
Agas occupying a volume of 673.0 ml at a pressure of 0.894 atm is allowed to expand at constant temp...
Questions


Mathematics, 29.01.2021 02:30


History, 29.01.2021 02:30


English, 29.01.2021 02:30

Mathematics, 29.01.2021 02:30

Computers and Technology, 29.01.2021 02:30

Chemistry, 29.01.2021 02:30

Spanish, 29.01.2021 02:30


Mathematics, 29.01.2021 02:30

Mathematics, 29.01.2021 02:30


English, 29.01.2021 02:30

Mathematics, 29.01.2021 02:30




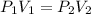
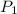 = initial pressure of gas= 0.894 atm
= initial pressure of gas= 0.894 atm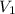 = initial volume of gas = 673.0 ml
= initial volume of gas = 673.0 ml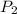 = final pressure of gas= 0.517 atm
= final pressure of gas= 0.517 atm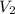 = final volume of gas = ?
= final volume of gas = ?