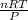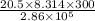Physics, 20.07.2019 02:20 khalilh1206
Acylinder with a movable piston contains 20.5 moles of a monatomic ideal gas at a pressure of 2.86 × 105 pa. the gas is initially at a temperature of 300 k. an electric heater adds 58600 j of energy into the gas while the piston moves in such a way that the pressure remains constant. it may you to recall that cp = 20.79 j/k/mole for a monatomic ideal gas, and that the number of gas molecules is equal to avagadros number (6.022 × 1023) times the number of moles of the gas. what is the temperature of the gas after the energy is added?

Answers: 1
Another question on Physics

Physics, 21.06.2019 23:30
Abeam of electrons with of wavelength of 7.5 x 10-6 m is incident on a pair of narrow rectangular slits separated by 0.75 mm. the resulting interference pattern is projected onto a screen 10.0 m from the slits. what is the separation of the interference maxima in the resulting interference pattern?
Answers: 2

Physics, 22.06.2019 05:00
Which is the best predictor of the radioactive nature of an isotope? o the proton-to-electron ratio the neutron-to-proton ratio o the neutron-to-electron ratio the electron-to-proton ratio
Answers: 1


You know the right answer?
Acylinder with a movable piston contains 20.5 moles of a monatomic ideal gas at a pressure of 2.86 ×...
Questions









Physics, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31

History, 13.11.2019 07:31


Mathematics, 13.11.2019 07:31


Mathematics, 13.11.2019 07:31



Mathematics, 13.11.2019 07:31

History, 13.11.2019 07:31