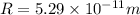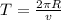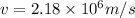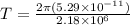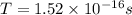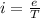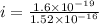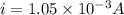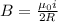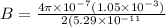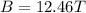Physics, 24.07.2019 16:10 itaheart101
In a simple picture of the hydrogen atom, the electron moves in circular orbits around the central proton attracted by the coulomb force. the lowest (n = 1) energy orbit that is allowed for the electron is at a radius of 5.29 × 10–11 m . calculate the magnetic field strength at the proton due to the orbital motion of the electron in the n = 1 state.

Answers: 2
Another question on Physics

Physics, 22.06.2019 20:10
On a frictionless air track, a blue glider with mass 0.200 kg is moving to the right with a speed of 8.00 m/s. it strikes a red glider that has mass 0.600 kg and that is initially at rest. after the collision, the blue glider is moving to the left with a speed of 3.00 m/s.(a) what are the magnitude and direction of the velocity of the red glider after the collision? (b) is this collision elastic?
Answers: 1

Physics, 23.06.2019 00:00
Which one of the following represents the reduced forms of the two major electron carriers? nadh and fadh2 nad+ and fadh2 nad+ and fad nadh and fad
Answers: 3

Physics, 23.06.2019 01:30
Relative to the ground below, how many joules of potential energy does a 1000-kg boulder have at the top of a 5-m ledge?
Answers: 1

Physics, 23.06.2019 02:00
If a nissan titan has a mass of 2,300 kilograms how many grams is that
Answers: 1
You know the right answer?
In a simple picture of the hydrogen atom, the electron moves in circular orbits around the central p...
Questions



Mathematics, 27.08.2020 20:01

History, 27.08.2020 20:01

Mathematics, 27.08.2020 20:01

Mathematics, 27.08.2020 20:01




Social Studies, 27.08.2020 20:01



Mathematics, 27.08.2020 20:01

History, 27.08.2020 20:01


Mathematics, 27.08.2020 20:01

Mathematics, 27.08.2020 20:01