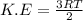Evaporation of sweat cools the skin because
a. liquids reduce the average kinetic energy of a...

Physics, 05.08.2019 19:30 InfinityVicky
Evaporation of sweat cools the skin because
a. liquids reduce the average kinetic energy of a surface by slowing down molecular movement
b. liquid water is always at a lower temperature than solid substances under the same conditions
c. the molecules with the highest energy evaporate first, lowering the temperature of the sample
d. the movement of the molecules during evaporation lowers the skin's temperature

Answers: 1
Another question on Physics

Physics, 22.06.2019 04:00
Determine the maximum r-value of the polar equation r =3+3 cos 0
Answers: 1

Physics, 22.06.2019 23:00
Which of the following describes how a microwave heats food
Answers: 2

Physics, 23.06.2019 01:30
)consider two positively charged particles, one of charge q0 (particle 0) fixed at the origin, and another of charge q1 (particle 1) fixed on the y-axis at (0,d1,0). what is the net force f⃗ on particle 0 due to particle 1?
Answers: 2

You know the right answer?
Questions

History, 12.12.2019 18:31



Mathematics, 12.12.2019 18:31



Mathematics, 12.12.2019 18:31


Mathematics, 12.12.2019 18:31

History, 12.12.2019 18:31

Mathematics, 12.12.2019 18:31


Mathematics, 12.12.2019 18:31

Mathematics, 12.12.2019 18:31

History, 12.12.2019 18:31


English, 12.12.2019 18:31

Mathematics, 12.12.2019 18:31