quantum mechanics or quantum physics is physics of very small objects (particles) and physical entities.
quantum physics has some parallels to our macro world and some pretty unique ideas which theoretically and mathematically proven to be true even though we will never be able to access them because of our size.
there are many types of particles: subatomic, elementary etc.
subatomic means they are smaller than atom, elementary means they are at the very basis of everything.
subatomic particles are for eg. protons, neutrons but not electrons.
elementary particles are categorised into 3 groups by what they do.
these 3 groups are quarks, leptons and bosons.
light for example is a particle more precisely a photon a part of leptons.
proton (subatomic particle) is made out of 2 up quarks and 1 down quark each of these has a fractional charge like  . (i crazy)
. (i crazy)
most famous scientist who contributed to this physics are:
max planckalbert einsteinerwin schrödingermany
the topic itself us understand a lot and will probably be vastly used in the future (quantum computers, understanding the big bang,
we also must not forget quantum effects like:
superposition (where 1 thing can be this and that at the same time),entanglement (where two particles are seemingly connected by some sort of unknown (or known) force, energy, matter (i know all that can be the same at least it was at the beginning)
and there is more. but probably too much for this answer to be consistent due to my average understanding of this topic.
hope this you understand quantum mechanics a little more. i'm not an expert in the topic (so don't be like wth if you are an expert) but i'm very interested in it.
anyways quantum physics is a very hard topic (harder than physics (perhaps)) therefore searching for every information there is about it and learning it is unfortunately impossible (you can only specialise into 1 - 3 things or more (perhaps
have fun and i wish you luck!
r3t40



























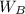 =10 g
=10 g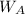 =90 g
=90 g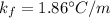
 C
C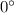 C
C