
Physics, 12.08.2019 17:20 graymonky12
Anegative change in entropy indicates . the products have a greater number of available energy microstates than the reactants. b. the products have a smaller number of available energy microstates than the reactants

Answers: 1
Another question on Physics

Physics, 22.06.2019 03:50
A30 kg weight lies on top of a massless piston of area a = 0.01 m2 the exterior air is at a (constant) p =1 atm and t = 27 c. the interior gas is 0.4 moles of (ideal) n2 and it has initial temperature 27.00 degrees c. 1. what is the initial pressure in the interior? a. 29.4 kpa b. 130.7 kpa c. 101.3 kpa the next three questions concern what happens when an amount of heat q is slowly added to the interior, raising the piston by 1 mm and raising the interior temperature to 27.40 c
Answers: 3


Physics, 22.06.2019 17:10
What causes the development of most clouds and precipitation in the atmosphere?
Answers: 1

Physics, 22.06.2019 22:10
M1 = 2.8 kg, m2 = 6.72 kg, m3 = 11.2 kg, byas in .is ,is .toof m3 it 0.91 m. (in m/s)
Answers: 3
You know the right answer?
Anegative change in entropy indicates . the products have a greater number of available energy micro...
Questions

English, 30.04.2021 22:00

Business, 30.04.2021 22:00

Mathematics, 30.04.2021 22:00

Mathematics, 30.04.2021 22:00


History, 30.04.2021 22:00


Mathematics, 30.04.2021 22:00



Mathematics, 30.04.2021 22:00





Mathematics, 30.04.2021 22:00

Biology, 30.04.2021 22:00


Mathematics, 30.04.2021 22:00

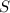 is a thermodynamic quantity defined as a criterion to predict the transformation of thermodynamic systems.
is a thermodynamic quantity defined as a criterion to predict the transformation of thermodynamic systems.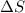 is matematically expressed as:
is matematically expressed as: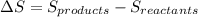 (1)
(1)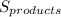 is the entropy of the products of the reaction
is the entropy of the products of the reaction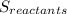 is the entropy of the reactants of the reaction
is the entropy of the reactants of the reaction

