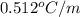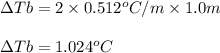Physics, 19.08.2019 19:30 ashleyvalles16
If you have a 1.0 m aqueous solution of nacl, by how much will it increase the water’s boiling point, if kb = 0.512 °c/m? in other words, what is the boiling point elevation (increase)?

Answers: 2
Another question on Physics

Physics, 22.06.2019 16:40
An owl dives toward the ground with a constant velocity of 4.40 m/s at 53.0° below the horizontal. the sun is directly overhead and casts a shadow of the owl directly below it. what is the speed (in m/s) of its shadow on level ground?
Answers: 3

Physics, 22.06.2019 17:00
Abowling ball rolling down the lane toward the pins has gravitational potential energy. a. no b. a lot of c. a little
Answers: 2

Physics, 22.06.2019 17:30
Frequency of electromagnetic waves that a radio station is assigned is called
Answers: 1

Physics, 22.06.2019 19:30
Assume that two of the electrons at the negative terminal have attached themselves to a nearby neutral atom. there is now a negative ion with a charge -2e at this terminal. what are the electric potential and electric potential energy of the negative ion relative to the electron? the electric potential and the electric potential energy are both twice as much. the electric potential is twice as much and the electric potential energy is the same. the electric potential is the same and the electric potential energy is twice as much. the electric potential and the electric potential energy are both the same. the electric potential is the same and the electric potential energy is increased by the mass ratio of the oxygen ion to the electron. the electric potential is twice as much and the electric potential energy is increased by the mass ratio of the oxygen ion to the electron.
Answers: 3
You know the right answer?
If you have a 1.0 m aqueous solution of nacl, by how much will it increase the water’s boiling point...
Questions

Social Studies, 23.12.2021 14:10

English, 23.12.2021 14:10

Mathematics, 23.12.2021 14:10


Social Studies, 23.12.2021 14:10


SAT, 23.12.2021 14:20





Mathematics, 23.12.2021 14:20


Mathematics, 23.12.2021 14:20






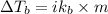
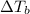 = change in boiling point = ?
= change in boiling point = ?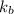 = boiling point constant =
= boiling point constant =