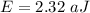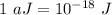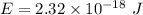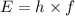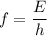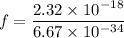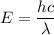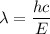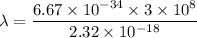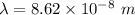Answers: 2
Another question on Physics

Physics, 21.06.2019 16:30
Picture a all traveling at a constant speed around the inside of a circular structure. is the ball accelerating? explain your answer
Answers: 3

Physics, 21.06.2019 20:20
Two friends are having a conversation. anna says a satellite in orbit is in freefall because the satellite keeps falling toward earth. tom says a satellite in orbit is not in freefall because the acceleration due to gravity is not 9.80 m/2 . who do you agree with and why?
Answers: 1

Physics, 22.06.2019 02:30
Mass (kg) force (n) 5 25 10 50 15 75 20 100 a student was trying to find the relationship between mass and force. he placed four different masses on a table and pulled them using a spring scale. the table shows the different masses used in the experiment and the force required to pull each mass. the student concluded that more force was required to pull heavier objects. what comment would you make regarding his conclusion? a) no clear relation can be observed between mass and force from the data. b) there is a direct proportion between the mass and force listed in the table. c) gravity should have been taken into account while performing the experiment. d) there is an inverse proportion between the mass and force listed in the table.
Answers: 2

Physics, 22.06.2019 11:10
Avolcano erupts next to a grassland area in a valley. a. describe three ways in which material released by the volcano could impact the grassland area. b. describe three ways in which the grassland ecosystem could recover after a volcanic eruption.
Answers: 3
You know the right answer?
The first ionization energy of a nitrogen atom is 2.32 aj (attojoules). what is the frequency and wa...
Questions


English, 04.11.2019 22:31


Mathematics, 04.11.2019 22:31

History, 04.11.2019 22:31

History, 04.11.2019 22:31

History, 04.11.2019 22:31









History, 04.11.2019 22:31



Mathematics, 04.11.2019 22:31

Mathematics, 04.11.2019 22:31