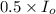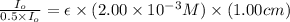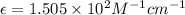Answers: 2
Another question on Physics

Physics, 22.06.2019 10:00
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 14:40
On a geologic map, if the contacts between sedimentary rock units form a bull’s-eye pattern of concentric circles, with the youngest unit in the center, the underlying structure is a(n)
Answers: 3

Physics, 23.06.2019 08:00
Use henry's law and the solubilities given below to calculate the total volume of nitrogen and oxygen gas that should bubble out of 1.7 l of water upon warming from 25 ˚c to 50 ˚c. assume that the water is initially saturated with nitrogen and oxygen gas at 25 ˚c and a total pressure of 1.0 atm. assume that the gas bubbles out at a temperature of 50 ˚c. the solubility of oxygen gas at 50 ˚c is 27.8 mg/l at an oxygen pressure of 1.00 atm. the solubility of nitrogen gas at 50 ˚c is 14.6 mg/l at a nitrogen pressure of 1.00 atm. assume that the air above the water contains an oxygen partial pressure of 0.21 atm and a nitrogen partial pressure of 0.78 atm.
Answers: 2

Physics, 23.06.2019 09:30
Why is the basic problem of economic,scarcity, a universal problem?
Answers: 2
You know the right answer?
A2.00 x10-3 m solution of a compound absorbs 50% of the light of a certain wavelength passing throug...
Questions




Business, 14.08.2019 08:30

History, 14.08.2019 08:30

Mathematics, 14.08.2019 08:30


Biology, 14.08.2019 08:30





Mathematics, 14.08.2019 08:30




Mathematics, 14.08.2019 08:30



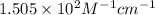

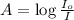
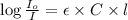
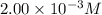
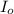 = incident light
= incident light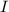 = transmitted light
= transmitted light = molar absorptivity coefficient = ?
= molar absorptivity coefficient = ?