Physics, 05.09.2019 17:10 zahradawkins2007
You have 1.50 kg of water at 28.0∘ in an insulated container of negligible mass. you add 0.700 kg of ice that is initially at -21.0 ∘c. assume that no heat exchanges with the surroundings.
(a) after the thermal equilibrium has been reached, has all the ice melted?
(b) if all of the ice is melted, what is the final temperature of the water in the container? if some ice remains, what is the final temperature of the water in the container, and how much ice remains?

Answers: 2
Another question on Physics

Physics, 21.06.2019 21:00
What can be found in every skeletal muscle? a. nerves, bones, cartilage, and connective tissue b. tendons, cartilage, nerves, and blood vessels c. muscle fibers, nerves, connective tissue, and blood vessels d. tendons, nerves, blood vessels, and bones
Answers: 1

Physics, 22.06.2019 04:00
Which configuration would produce an electric current? a) rotate a coil of copper wire between two magnets. b) connect a wire between a copper and zinc strip sitting in a beaker of water. c) connect a wire to the (+) positive end of a battery and the other end to a light bulb. d) connect a wire to the negative end of a battery and the other end to a light bulb.
Answers: 2

Physics, 22.06.2019 17:30
Convection currents are caused by differences in what two things?
Answers: 1

Physics, 22.06.2019 18:00
Efficiency is the ratio of to for a system a)power in to power in b)power in to power out c) power out to power out d) power out to power in
Answers: 2
You know the right answer?
You have 1.50 kg of water at 28.0∘ in an insulated container of negligible mass. you add 0.700 kg of...
Questions

History, 28.02.2020 03:25

Chemistry, 28.02.2020 03:25



Spanish, 28.02.2020 03:25

Mathematics, 28.02.2020 03:25

English, 28.02.2020 03:25

Mathematics, 28.02.2020 03:25








Mathematics, 28.02.2020 03:26

Mathematics, 28.02.2020 03:26

Mathematics, 28.02.2020 03:26

Mathematics, 28.02.2020 03:26

Biology, 28.02.2020 03:26

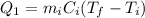
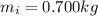 is the mass of the ice
is the mass of the ice is the heat specific capacity of ice
is the heat specific capacity of ice is the final temperature of the ice
is the final temperature of the ice is the initial temperature
is the initial temperature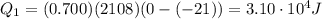

 is the mass of the water
is the mass of the water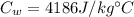 is the heat specific capacity of water
is the heat specific capacity of water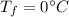 is the final temperature at equilibrium
is the final temperature at equilibrium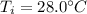 is the initial temperature of the water
is the initial temperature of the water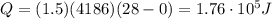
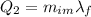
 is the latent heat of fusion of ice
is the latent heat of fusion of ice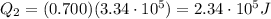
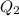 is larger than Q: this means that not all the ice melts.
is larger than Q: this means that not all the ice melts. 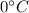
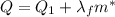
 is the mass of ice that has melted. Solving for this variable, we find:
is the mass of ice that has melted. Solving for this variable, we find: