Physics, 14.09.2019 06:30 deannajd03
When 19.3 j was added as heat to a particular ideal gas, the volume of the gas changed from 56.7 cm^3 to 104 cm^3 while the pressure remained constant at 0.947 atm. (a) by how much did the internal energy of the gas change? if the quantity of gas present is 1.97 x 10^-3 mol, find the molar specific heat of the gas at (b) constant pressure and (c) constant volume.

Answers: 3
Another question on Physics

Physics, 22.06.2019 00:00
Name three different units of energy used to measure heat and describe what type of situations each is usually used.
Answers: 1


Physics, 22.06.2019 20:30
Atypical jetliner lands at a speed of 146 mi/h and decelerates at the rate of (10.4 mi/h)/s. if the jetliner travels at a constant speed of 146 mi/h for 1.5 s after landing before applying the brakes, what is the total displacement of the jetliner between touchdown on the runway and coming to rest?
Answers: 2

Physics, 22.06.2019 22:30
Use the illustration to describe how the electromagnetic spectrum changes as its frequency moves from radio waves to higher energy gamma waves in terms of wavelength and amplitude.
Answers: 1
You know the right answer?
When 19.3 j was added as heat to a particular ideal gas, the volume of the gas changed from 56.7 cm^...
Questions

Mathematics, 21.02.2020 06:55

Mathematics, 21.02.2020 06:55

English, 21.02.2020 06:55

Mathematics, 21.02.2020 06:56


Mathematics, 21.02.2020 06:56

Mathematics, 21.02.2020 06:56




Mathematics, 21.02.2020 06:57



Mathematics, 21.02.2020 06:57

Mathematics, 21.02.2020 06:57

Computers and Technology, 21.02.2020 06:57


Mathematics, 21.02.2020 06:58

Law, 21.02.2020 06:58

History, 21.02.2020 06:58

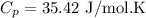
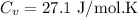
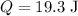 Initial volume of the gas,
Initial volume of the gas,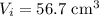 Final volume of the gas,
Final volume of the gas, 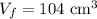 Constant pressure of the gas,
Constant pressure of the gas,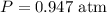 Number of moles of the gas,
Number of moles of the gas,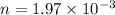
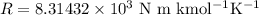
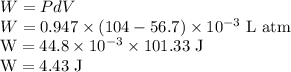
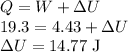
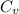 be the molar specific heat of the gas at constant volume given by
be the molar specific heat of the gas at constant volume given by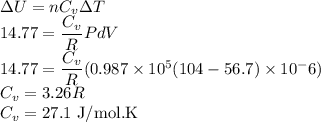
 be the molar specific heat of the gas at constant pressure given by
be the molar specific heat of the gas at constant pressure given by