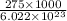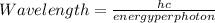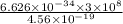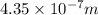Physics, 16.09.2019 20:30 nayiiii1874
The energy required to dislodge electrons from sodium metal via the photoelectric effect is 275 kj/mol . what wavelength of light, in nanometers, has sufficient energy per photon to dislodge an electron from the surface of sodium? express the wavelength in nanometers to three significant figures.

Answers: 1
Another question on Physics


Physics, 22.06.2019 15:00
Steelhead trout migrate up stream to spawn.occasionally they need to leap up small waterfalls to continue their journey. fortunately, steelhead are remarkable jumpers, capable of leaving the water at a speed of 8.0 m/s. a. what is the maximum height a steelhead trout can jump? b.leaving the water at 8.0 m/s, the trout lands on top of the water fall 1.8 m high. how long was it in the air?
Answers: 2


Physics, 22.06.2019 18:00
Directions: count the number of atoms. ar co2 na3po4 so3 nac2h3o2
Answers: 1
You know the right answer?
The energy required to dislodge electrons from sodium metal via the photoelectric effect is 275 kj/m...
Questions


Mathematics, 16.11.2020 19:50


Mathematics, 16.11.2020 19:50

Mathematics, 16.11.2020 19:50

Mathematics, 16.11.2020 19:50

Mathematics, 16.11.2020 19:50

Computers and Technology, 16.11.2020 19:50

Social Studies, 16.11.2020 19:50

Mathematics, 16.11.2020 19:50


Mathematics, 16.11.2020 19:50

English, 16.11.2020 19:50


Social Studies, 16.11.2020 19:50



Mathematics, 16.11.2020 19:50