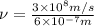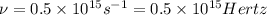Physics, 17.09.2019 01:00 xavierbanks802
Given that the energy difference between the ground state and the first excited electronic state (e) for the sodium atom is 3.373 × 10-19 j, calculate the frequency, , corresponding to a photon possessing this energy. next, calculate the wavelength (in nm) for this photon.

Answers: 2
Another question on Physics

Physics, 21.06.2019 14:40
If you go to a nutrition store and buy a supplement, you can count on the fact that it is pure and safe because supplements are regulated, controlled, and inspected by the fda.
Answers: 1

Physics, 22.06.2019 04:30
Asystem containing an ideal gas at a constant pressure of 1.22×10^5 pa gains 2140 j of heat. during the process, the internal energy of the system increases by 2320 j. what is the change in volume of the gas?
Answers: 3

Physics, 22.06.2019 04:30
Work out sian speed for the first 30 minutes of her journey. give your answer in km/h.
Answers: 1

Physics, 22.06.2019 06:00
Explain earth's motion, using the terms "precession" and "rotation" in your answer.
Answers: 1
You know the right answer?
Given that the energy difference between the ground state and the first excited electronic state (e...
Questions




Mathematics, 16.10.2020 21:01

Chemistry, 16.10.2020 21:01


Mathematics, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01






History, 16.10.2020 21:01


Mathematics, 16.10.2020 21:01




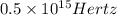
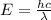
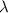 = wavelength of the wave
= wavelength of the wave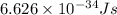
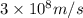
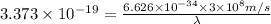
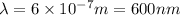
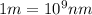
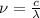
 = frequency of the wave
= frequency of the wave