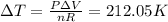When 25.9 j was added as heat to a particular ideal gas, the volume of the gas changed from 41.0 cm3 to 67.5 cm3 while the pressure remained constant at 1.08 atm. (a) by how much did the internal energy of the gas change? if the quantity of gas present is 1.66 x 10-3 mol, find the molar specific heat of the gas at (b) constant pressure and (c) constant volume.

Answers: 3
Another question on Physics

Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1

Physics, 22.06.2019 17:00
Simon is writing a story about an astronaut whose spacecraft has been boarded by space pirates. the astronaut has her lucky penny in her hand behind her back as the space pirates break into the control room. she has just locked the controls so that the ship is accelerating in the direction of the control room’s ceiling. simon wants the astronaut to use the penny to hit a button on the control panel to turn off the lights and escape. the button is located a short distance behind and below the astronaut’s hands. how should simon use the theory of relativity to describe what the astronaut must do in order to hit the button?
Answers: 1

Physics, 22.06.2019 17:00
Since the energy was not raising the temperature of the water during these segments, what was the energy used to do instead? your answer must include an explanation of what is happening to the molecules.
Answers: 3

Physics, 22.06.2019 23:00
Acommon technique in analysis of scientific data is normalization. the purpose of normalizing data is to eliminate irrelevant constants that can obscure the salient features of the data. the goal of this experiment is to test the hypothesis that the flux of light decreases as the square of the distance from the source. in this case, the absolute value of the voltage measured by the photometer is irrelevant; only the relative value conveys useful information. suppose that in part 2.2.2 of the experiment, students obtain a signal value of 162 mv at a distance of 4 cm and a value of 86 mv at a distance of 5.7 cm. normalize the students' data to the value obtained at 4 cm. (divide the signal value by 162.) then calculate the theoretically expected (normalized) value at 5.7 cm.
Answers: 2
You know the right answer?
When 25.9 j was added as heat to a particular ideal gas, the volume of the gas changed from 41.0 cm3...
Questions

Social Studies, 24.11.2019 05:31

Biology, 24.11.2019 05:31

Biology, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31



History, 24.11.2019 05:31

English, 24.11.2019 05:31


Chemistry, 24.11.2019 05:31





History, 24.11.2019 05:31



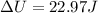
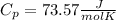
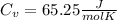
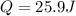
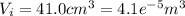
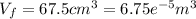
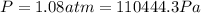
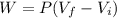 .
.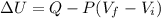 ⇒
⇒ 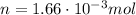
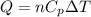 ⇒
⇒ 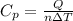
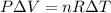 ⇒
⇒