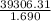Physics, 20.09.2019 23:20 laqu33n021
Chemical engineer studying the properties of fuels placed 1.690 g of a hydrocarbon in the bomb of a calorimeter and filled it with o2 gas. the bomb was immersed in 2.550 l of water and the reaction initiated. the water temperature rose from 20.00°c to 23.55°c. if the calorimeter (excluding the water) had a heat capacity of 403 j/k, what was the heat of reaction for combustion (qv) per gram of the fuel? (d for water = 1.00 g/ml; c for water = 4.184 j/g·°c.) enter your answer in scientific notation.

Answers: 2
Another question on Physics

Physics, 21.06.2019 21:20
Which of the following explains why it took so long for the public to accept the negative health effects of smoking? a)people were waiting for counter reasons, and those were presented.b)scientists had not completely finalized their results, which took 30 years.c)cigarettes were cheaply available and media channels were very inefficient.d)cigarette manufacturers were trying hard to bring an alternative into the market
Answers: 3



Physics, 22.06.2019 23:30
An astronaut on the moon throws a baseball upward. the astronaut is 6 ft, 6 in. tall, and the initial velocity of the ball is 3030 ft per sec. the height s of the ball in feet is given by the equation s equals negative 2.7 t squared plus 30 t plus 6.5s=−2.7t2+30t+6.5, where t is the number of seconds after the ball was thrown. complete parts a and b.
Answers: 2
You know the right answer?
Chemical engineer studying the properties of fuels placed 1.690 g of a hydrocarbon in the bomb of a...
Questions


Mathematics, 03.12.2020 21:20



Mathematics, 03.12.2020 21:20

Mathematics, 03.12.2020 21:20

Mathematics, 03.12.2020 21:20


Mathematics, 03.12.2020 21:20


Mathematics, 03.12.2020 21:20

French, 03.12.2020 21:20


History, 03.12.2020 21:20

English, 03.12.2020 21:20

Mathematics, 03.12.2020 21:20

Mathematics, 03.12.2020 21:20


Mathematics, 03.12.2020 21:20